EFFORTS
We are interested in looking at proteins that are involved in human disease. By understanding the way they fold, move and interact with other proteins we can better develop therapeutics and treatments to modulate their activity. We are specifically interested in membrane proteins, those proteins that reside in the lipid membranes of cells. We are also looking at how post-translational modification, e.g. glycosylation, affects the physical properties of these proteins.
Glycoproteins
Glycoproteins are a large class of proteins, taking part in nearly every biological process. They participate in the immune system as antibodies and as factors in the major histocompatibilty complex interacting with T cells as part of a the adaptive immune response. They are also involved in white blood cell recognition, cell growth, differentiation, cell-cell interactions and protein folding. Glycoproteins are also indicators for various cancers. A large number of important glycoproteins are integral membrane proteins. They can be found in the lipid bilayers that make up the plasma membrane and the membranes of organelles. In the laboratory of Dr. Cook a combination of solution and solid-state NMR are employed to study the effects of glycosylation on structure, dynamics and the interactions of these important proteins. Understanding these properties is an incredibly important component to the development of treatments of human disease involving glycoproteins
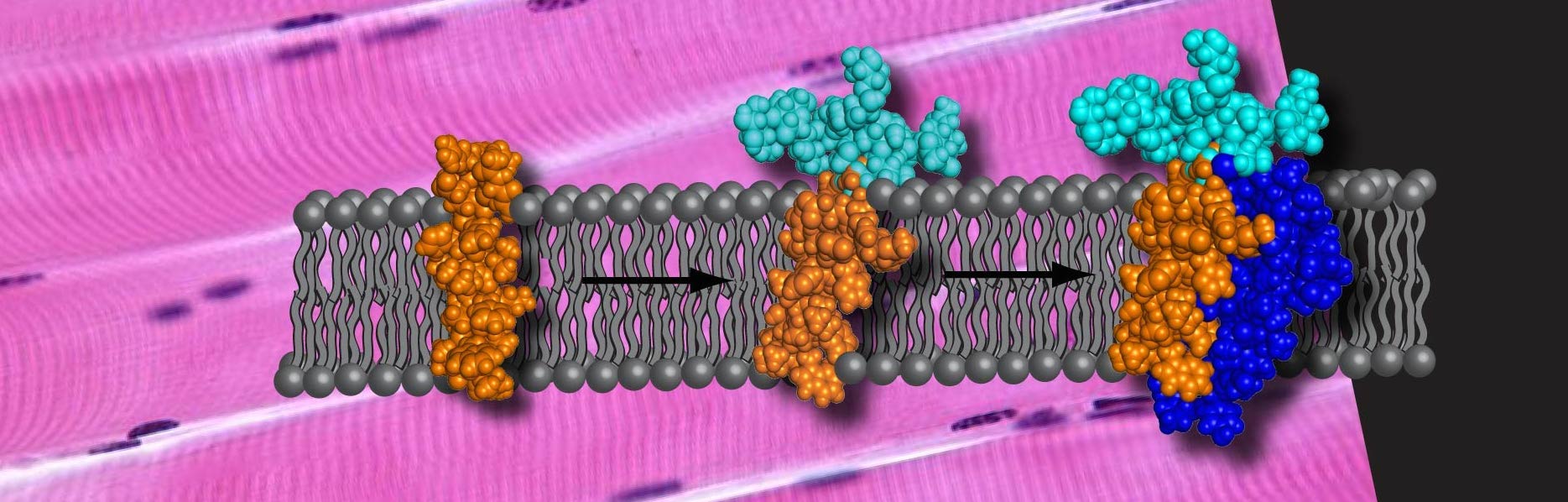
Dystrophin Complex
A large percentage of glycoproteins are membrane proteins. Sarcoglycans are a set of these important proteins. They are responsible for maintaining the integrity of the muscle fiber sarcolemma by connecting the cytoskeleton of the muscle fiber to the extracellular matrix. Very little is known about the structure and dynamics of this set of proteins and of membrane glycoproteins overall. These proteins are not soluble in aqueous environments making them difficult to study by most spectroscopic methods. We have been able to overexpress full-length γ-sarcoglycan in e. coli in an effort to learn more about the structure, dynamics and interactions of this biologically important protein.
Syndecans
Each syndecan has a short cytoplasmic domain, a highly conserved single spanning transmembrane domain, and a large extracellular domain with attachment sites for three to five heparin sulfates or chondroitin sulfates. The syndecan family is involved in the regulation of angiogenesis and cell proliferation, cell-to-cell interaction and cell adhesion through the activation of growth factors. Both syndecan-1 and syndecan-4 have been closely associated with tumor progression. Syndecan-1 was shown to be highly overexpressed on the cell surface in aggressive tumor cells and is associated with poor prognosis. Studies have also demonstrated that the shedding of the syndecan-1 soluble ectodomain inhibits FGF2-induced cell proliferation and is also a potent stimulator for melanoma tumor growth and metastasis. Syndecan-1 is used clinically as a blood plasma tumor marker, through solution NMR spectroscopy. It is thought that the presence of the highly conserved cytoplasmic C1 domain promotes sydecan dimerization. Our computational studies predict that the large ectodomain of syndecan-1 is intrinsically disordered but may adopt a more defined secondary structure upon ligand binding. Despite the significance of these proteins, very little is known about the structure and dynamics of these proteins. To date, only the structure of the cytoplasmic domain of syndecan-4 has been solved. We will use both solution and solid-state NMR to characterize these important proteins and look at the effect that glycosylation has on their properties.
Structure, Dynamics
By determining the structure and dynamics of these proteins we will better understand how they perform their function in biology. Very little is known about membrane glycoproteins and our studies will help other researchers that are working on glycoproteins to better predict their structures and behaviors, leading to a broader knowledge about this important family of proteins.
Protein-Protein and Drug-Protein Interactions
Interactions are another important part of our research. We can use interaction studies to determine how these proteins perform their functions. In certain disease states the blocking of these interactions may be a way to inhibit harmful interactions of proteins that lead to disease. By knowing the precise locations on the protein that are involved in these interactions, drugs can be made and tailored to bind to these sites and block the protein-protein interactions.